Centering Patients in Long COVID Research
Patient advocates are cautiously optimistic that forthcoming studies will track their concerns and lead to treatments.
In 2021, Robert DeRosa sought treatment for crushing fatigue, unexplained pains, loss of teeth, gastrointestinal distress, and other symptoms that had persisted for nearly a year following a COVID-19 infection. His physician, a pulmonologist working at a newly set up long COVID treatment center, told him he was just deconditioned. “He was super dismissive and condescending,” DeRosa told me in September. “I was like, ‘Dude, I’m a marathon runner, I know how to train myself.’ My resting heart rate was over 100 and before I got sick it was 40, 50.”
DeRosa says that at the time of his appointment, he was so fatigued and cognitively scrambled that he couldn’t remember his sister’s name or type on a keyboard; seeking work (he had been a teacher) was out of the question.
This is typical of many people experiencing long COVID, who often report that they now work less or can no longer work at all. Mapping disability rates caused by long COVID is challenging, but data from the US Bureau of Labor Statistics show that disability among working-age Americans soared over 30% since the start of the pandemic, from around 6.4 million in 2020 to 8.4 million in 2024. A National Academies of Sciences, Engineering, and Medicine (NASEM) report published last June reported that 3.4% of US adults and 0.5% of US children were experiencing long COVID at the time of a 2022 survey. Noting that the data are “rapidly evolving,” the NASEM report, commissioned by the Social Security Administration, also found that many patients with long COVID symptoms 3 months after the initial infection showed improvement by 12 months, but that progress often plateaued or slowed after 12 months.
DeRosa’s experience in his doctor’s office is also typical. From early in the pandemic, patients have reported that care has been fragmented, difficult to access, and often demoralizing. Although many long COVID clinics popped up fairly quickly, they were often staffed by rehabilitation or pulmonary medicine specialists unfamiliar with problems common in infection-associated chronic illnesses, such as chronic fatigue and dysfunction of nerves that control autonomic processes like sweating and heart rate.
As long COVID patients have learned, often the best care I could find came via suggestions from other patients, not physicians.
“Long COVID patients suffer from the double burden of feeling unwell and being unseen in clinical spaces,” Abigail Dumes, a medical anthropologist at the University of Michigan, said at a June press conference on the report. DeRosa found validation, fellowship, and suggestions for managing his symptoms in Survivor Corps, an early group of long COVID patient advocates online.
The lack of quality health care for long COVID patients mirrors the neglect faced by populations with similar infection-triggered chronic illnesses, most notably myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Surveys have found that over half of people with that diagnosis say it began with some kind of infection. But very few physicians in the United States are literate in ME/CFS care, and seeing a specialist is difficult or nearly impossible. It took me, an experienced science journalist, more than a year to receive a diagnosis of ME/CFS when my health suddenly declined after a high fever. As long COVID patients have learned, often the best care I could find came via suggestions from other patients, not physicians.
In fact, patients’ visibility and voices are likely key to developing effective treatments for long COVID. Patients have published some of the most influential research in the field. In comments addressed to attendees of a National Institutes of Health (NIH) conference in September 2024, Ziyad Al-Aly of the US Veterans Administration in St. Louis, who has published several highly cited epidemiological studies of long COVID, decried how institutions like NIH have stuck to old ways of setting research priorities with little patient input. “Rule number one is ‘listen to patients.’ They have been right all along,” he says.
Being seen
Patients coined the term “long COVID” to describe a vast collection of health issues that range from annoying to disabling or even fatal.
Patients coined the term “long COVID” to describe a vast collection of health issues that range from annoying to disabling or even fatal. And it was Hannah Davis and Lisa McCorkell, both patients and cofounders of the Patient-Led Research Collaborative, who were the lead authors on a January 2023 article published in Nature Reviews Microbiology titled “Long COVID: Major Findings, Mechanisms, and Recommendations.” According to Altmetric, as of October 2024, the article had been accessed 1.5 million times, cited more than 1,500 times, and mentioned in 1,338 news articles and more than 50,000 social media posts. Of the nearly 500,000 biomedical articles of similar age, it is in the 99th percentile for impact. The publication continued a years-long, patient-driven campaign to advance discussions and research around long COVID.
Now patient perspectives are shaping further definitions of the disease. This past June, to help unify research and expand treatment efforts, NASEM published a broad definition of long COVID meant to be adopted by medical societies, research organizations, government agencies, and patient groups—anyone with a stake in the disease. The definition drew heavily on patient experiences, with several patients and advocates serving on the committee. (The new definition has not yet been extensively deployed for epidemiology and was not used to arrive at current prevalence estimates.)
“Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems,” the definition begins. It goes on to say that long COVID can appear after mild, moderate, or severe initial infections; onset can be delayed; and that diagnosing and managing long COVID should not require a positive COVID test.
Researchers and advocates largely applauded the definition, which is subject to revision as knowledge advances. One member of the definition committee is Karyn Bishof, a former firefighter and paramedic who became ill with acute and then long COVID in March 2020. At a webinar announcing the new definition, she said it “destigmatizes long COVID and validates the experience of millions.”
The committee’s work should help more people get access to medical care. “They were looking to be very inclusive, which from a clinical strategy standpoint is crucial,” says David Putrino, a rehabilitation medicine specialist who manages a busy clinic at Mount Sinai Hospital in New York City that treats long COVID and similar disorders and who reviewed the definition before publication. “So many people are being denied disability benefits, being denied insurance coverage for care,” he explained to me. “You can help the most people the fastest by being inclusive.”
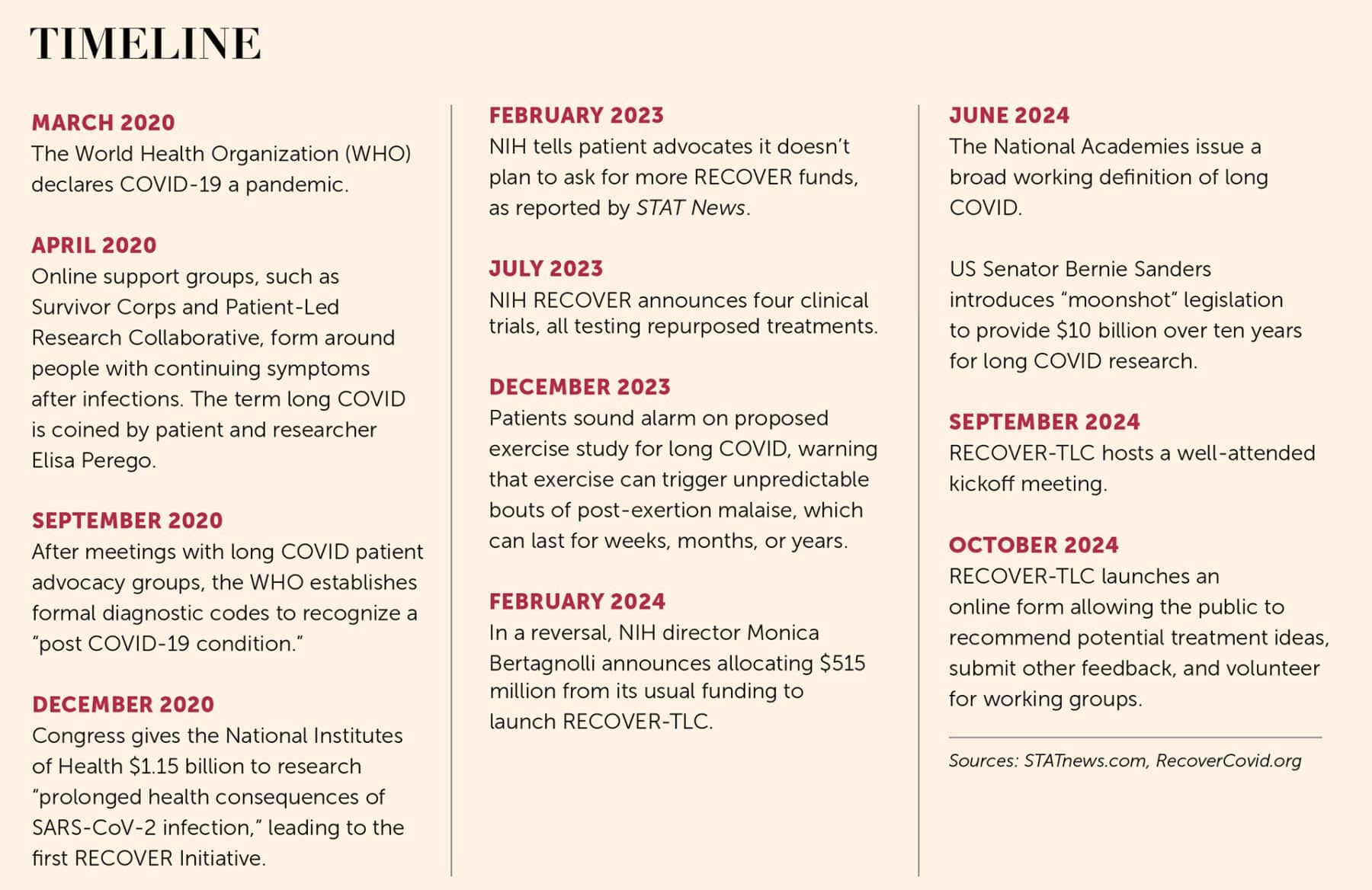
Two initiatives named RECOVER, one with TLC
Patient advocates hope that the kind of collaboration that has contributed to this definition of the disease will now infuse research into clinical treatments, particularly a $515 million project launched in September 2024 by NIH and set to run until 2028. An estimated 200 people attended the September 2024 launch meeting in person, with another 1,300 attending online—an indication of the great interest in the new effort, which was introduced as Researching COVID to Enhance Recovery–Treating Long COVID (RECOVER-TLC).
Patient advocates hope that the kind of collaboration that has contributed to this definition of the disease will now infuse research into clinical treatments.
RECOVER-TLC is the revamped second phase of a $1.1 billion effort launched in 2021, also called RECOVER, which patient advocates say failed to meet its goals in terms of information and treatment ideas, in large part because it lacked meaningful input from patients and researchers with expertise in infection-associated chronic illnesses. The original RECOVER focused on observational studies: watching large cohorts, documenting the variable and heterogeneous course of long COVID, and running standard lab work. And while RECOVER scientists invited patients to advise them, these patient advisors regularly said they felt their ideas were shunned and their advice went unheeded. “Patients haven’t had a lot of input” into the initial RECOVER effort, Jaime Seltzer, scientific director of #MEAction, an advocacy group for people with ME/CFS and long COVID, told me.
In 2023, when RECOVER finally announced its first treatment trials, advocates were further incensed: Instead of testing medicines, the first trials would test cognitive and physical exercises—which have both been shown to be harmful to people with a specific symptom called post-exertional malaise (PEM), common with long COVID and ME/CFS. In people with PEM, symptoms flare and worsen after physical, cognitive, or emotional exertion. At its worst, PEM can be totally debilitating, leaving patients bedbound in darkened rooms, unable to tolerate any stimuli. Such episodes can be unpredictably long, extending for hours, days, months, or even permanently. (After patients raised concerns, trial administrators modified enrollment criteria to exclude anyone diagnosed with PEM.)
More than five years after the COVID-19 pandemic began and four years after RECOVER launched, no treatments for long COVID have received approval by the US Food and Drug Administration (FDA). In 2023 and 2024, the initiative launched studies of a handful of behavioral training programs as well as other clinical trials investigating whether drugs approved for other conditions could successfully treat long COVID. In contrast, the first therapeutic to receive emergency FDA approval for treating acute COVID-19 appeared in October 2020, only seven months after the first documented cases in the United States.
At the RECOVER-TLC meeting, Leora Horwitz, a prominent RECOVER researcher at New York University’s Langone Health, defended NIH’s initial approach: “Long COVID is a new entity, and we are fully dependent on observational studies to tell us what it is.” Such observational data point to “the right treatment targets” and help unravel the underlying biology of long COVID, she said. She added that the RECOVER studies to date have shown that “this is not just fatigue,” validating that long COVID can include neurological disturbances, cognitive dysfunction, and many other symptoms.
Of course, patients have asserted that long COVID is not just fatigue since the spring of 2020, and advocates for other neglected infection-associated chronic conditions have repeatedly pointed out that neither the symptoms nor the variable course of illness nor the patterns of lab measurements observed in long COVID are new at all. To anyone familiar with chronic Lyme disease or ME/CFS, the similarities are obvious.
Advocates say RECOVER-TLC seems to be offering a more inclusive and encouraging path toward treatments than its predecessor. That first effort has an executive committee with over a dozen members and chairs drawn from three institutes. Also, according to an investigative report from the Sick Times and STAT News, the initial RECOVER tapped expertise in big data, such as genomics and electronic health records, rather than in relevant diseases. In contrast, RECOVER-TLC will be led by the National Institute of Allergy and Infectious Diseases (NIAID) and supported by the Foundation for the National Institutes of Health, the organizations that led public-private US efforts to develop vaccines and treatments during the pandemic.
Advocates worry that the past four years have shown that the biomedical research world has learned very little from decades of stigmatizing and neglecting ME/CFS.
#MEAction’s Seltzer celebrated the new organization, pointing out that NIAID has sponsored most past ME/CFS research and famously worked closely with HIV/AIDS patients in the 1990s. Lisa McCorkell of the Patient-Led Research Collaborative, which has conducted much of the foundational academic work on long COVID, told me over email that advocates were impressed by how attentive NIAID director Jeanne Marrazzo has been to their concerns.
Still, advocates worry that the past four years have shown that the biomedical research world has learned very little from decades of stigmatizing and neglecting ME/CFS. Since the 1970s, the syndrome has been underrecognized, underfunded, and underresearched. Until recently, many medical schools taught that the condition is psychosomatic—a misrepresentation refuted by the research literature, and an old story that also attached itself to long COVID like a bad odor. That neglect has led to a landscape with no FDA-approved treatments and woefully inadequate funding, with NIH spending only $13 million on ME/CFS in 2024 despite a 2015 Institute of Medicine estimate that up to 2.5 million Americans were living with the disorder.
Shifty disease
W. Ian Lipkin, a virologist at Columbia University who studies ME/CFS, encouraged RECOVER-TLC leaders during the kick-off meeting to become familiar with the ME/CFS literature. In particular, he advised that two key features of both diseases—cognitive dysfunction, also known as brain fog, and post-exertional malaise—should be intensively studied. He added that findings from long COVID work can feed back into helping ME/CFS patients: “It’s a two-way street.”
Though the search for ME/CFS treatments has been dishearteningly slow for patients, one lesson is that listening to patients can point the way forward. In 2016, I was part of a group that brought the lack of progress in ME/CFS to the attention of NIH’s then director Francis Collins. Collins responded by ordering a “deep phenotyping” study, promising that it would be followed by drug trials. I took part as a patient volunteer in 2017 and 2018, spending several weeks at the NIH Clinical Center. The COVID pandemic ended the research after just 17 patients had completed the protocol.
In early 2024, Clinical Center neurologist Avindra Nath and colleagues published the primary results of the study. More research papers are in the pipeline, but the main results were underwhelming. Nath used the data collected to develop a hypothesis that a “persistent antigen” causes immune and neurological dysfunction, but the scientists did not identify any specific candidates. Nath has since adapted the ME/CFS protocol for long COVID and deeply phenotyped a few dozen patients. He’s developing a survey instrument that he hopes will be widely adopted to categorize patients and predict effective treatments. Currently, he uses a narrow definition of long COVID: Patients must have chronic fatigue, brain fog, and autonomic dysfunction.
Like ME/CFS, a coterie of factors makes long COVID treatments hard to study: competing—or possibly complementary—theories of what’s gone wrong with the body, fluctuating symptoms, dozens of potential biomarkers but none validated in large studies, and unstable disease phenotypes, such as abnormal immune measurements that shift over time.
For his long COVID work, Putrino has bypassed NIH altogether, saying that the way the agency awards funding penalizes potentially transformative research in favor of low-risk, incremental progress.
Putrino says his clinic at Mount Sinai “deeply quantifies” patients via tests for co-infection, immune profiling, and more. “We take repeated measures on 30 to 40 people,” he says. “Then we look for responders and see what changed in their physiology to explain the response.” His clinic, infused with $10 million in philanthropic funding and renamed the Cohen Center for Recovery from Complex Chronic Illnesses in 2024, serves patients with long COVID, ME/CFS, and related conditions.
For his long COVID work, Putrino has bypassed NIH altogether, saying that the way the agency awards funding penalizes potentially transformative research in favor of low-risk, incremental progress—which he finds wholly inadequate for a disorder with such an urgent need for treatment. The traditional NIH model of trying to understand the pathobiology of a disorder before funding treatment trials leaves patients suffering without options, he says.
During September’s kickoff meeting for RECOVER-TLC, researchers repeatedly spoke of targeting subpopulations of patients to suss out which treatments might work for different symptom profiles and which biological measurements could be clinically useful. Patient advocates also called for smaller, rapid clinical trials, warning that devoting huge resources looking for a silver bullet to treat all patients is a fool’s errand, as long COVID is complex and heterogeneous. “Long COVID is not a single entity, [or] a single biomarker disease,” said Resia Pretorius, a biologist at Stellenbosch University in South Africa.
Other meeting participants expressed frustration with the state of knowledge to date. Lisa Purcell, who’d led development teams at Vir Biotechnologies for COVID-19 treatments before moving to Third Rock Ventures, listed looming scientific unknowns thwarting the hunt for treatments. “After a billion dollars [spent on RECOVER], we are no closer to finding out what types of drugs we need,” she pointed out.
Ominously, some drug trials for long COVID have already failed, including a study that gave patients a two-week course of Paxlovid, which is approved to treat acute COVID-19. A September 2024 report in Chemical & Engineering News detailed how several biotech companies have gone bankrupt or otherwise abandoned development of long COVID therapeutics.
After being rejected by NIH for a drug development grant, a small biotech company, BioVie, won a $12.6 million grant from the Department of Defense’s Congressionally Directed Medical Research Programs to test one of its drugs in treating neurological effects of long COVID. The Defense Department’s program was established in 1992 after breast cancer advocates, frustrated with NIH, convinced Congress to fund another outlet for high-risk, high-value research. BioVie’s drug, which is also being tested for Alzheimer’s and Parkinson’s diseases, targets neuroinflammation, which has been found in long COVID patients. But Penelope Markham, the company’s head of long COVID research, says barriers remain beyond funding a trial. “There’s no clear pathway at the FDA yet,” she told me, for demonstrating that a drug is effective against long COVID.
NIAID’s Marrazzo has said that the institute would organize a meeting with representatives from the FDA and the European Medicines Agency to develop a recommended set of end points for long COVID drug trials. As of November 2024, plans have not yet been announced.
Shortly after the RECOVER-TLC kickoff meeting, the Foundation for the National Institutes of Health launched a website where anyone can propose treatments they think should be tested. The suggestions will likely be myriad; one meeting participant showed slides listing more than 100 prescription medications, over-the-counter drugs, and dietary supplements that long COVID patients currently use to manage symptoms. So far, advocates said the launch of RECOVER-TLC has them cautiously hopeful that NIAID will fulfill its promises of meaningful patient engagement. Still, in a landscape with no FDA-approved treatments, a large gray literature of patient experiences guides millions of people seeking relief. Coached by the ME/CFS community, long COVID patients have discovered that, in the face of a shambolic biomedical response, their best resource for improving the quality of their lives is each other.
