To Speed New COVID Vaccines, Look to Patenting
Prioritizing collaboration and technology transfer could be key to providing global access to COVID-19 vaccines.
The COVID-19 pandemic has sparked extensive research activities to develop and manufacture vaccines and treatments to prevent infection or hasten recovery, and these efforts have set off debates about how best to enable access to these products. In previous health crises, such as HIV/AIDS, it was massive imitation enabled by relaxed approaches to intellectual property protection that allowed lifesaving drugs to reach so much of the world’s population. Achieving widespread access in the COVID context, however, will require overcoming a different set of challenges. This new context is best thought of as one where, for many essential products, reverse-engineering is difficult, technology transfer depends on collaboration, and fast and massive scaling up of production are high priorities. These conditions prompt fresh—and perhaps uncomfortable—thinking about the relationship between patents and access.
It’s become common wisdom in the “access to medicines” community that the way to increase access to pharmaceutical products is to reduce the legal barriers to market entry that patents impose, thereby increasing competition among multiple suppliers. If maximum access is the goal, it makes little sense to rely on single suppliers when there are hundreds of labs across the globe that can produce medically equivalent, high-quality versions of the same drugs. In this view, increasing competition should not just generate lower prices, but may also reveal new and better products, such as new formulations and combinations.
The logic of openness and competition became clear with the HIV/AIDS treatment campaign of the early 2000s, when supplies were expanded and prices reduced as a number of firms in India began providing nonpatented small-molecule drugs, the type of pills and capsules comprising most HIV/AIDS therapies. And today, during the COVID pandemic, it is commonly invoked.
In fact, relaxing patents to let multiple firms produce therapeutic agents is generally regarded as more essential during the COVID pandemic, with the goal being to maximize production. The global antipoverty charity Oxfam, in calling for a “people’s vaccine,” for example, declared: “If the goal is mass production of safe and effective vaccines, we need as many factories producing as fast as possible—without the need to ask for permission from large pharmaceutical corporations.” Likewise, when Costa Rica and the World Health Organization launched the COVID-19 Technology Access Pool (C-TAP), WHO’s director-general and Costa Rica’s president declared: “An open framework built around voluntarily shared information is not just good for science. It also will maximize the number of companies involved in producing in-demand technologies, thereby scaling up worldwide availability, lowering costs, and helping secure universal access.”
But the rapid spread of the COVID pandemic, the vast quantities of products needed, and the complexity of producing highly engineered products should raise the question of whether relaxing patents might achieve needed goals. For some COVID products (vaccines in particular), the principle barriers to access will be related to their production. In such cases, the primary focus should be on promoting the transfer of knowledge and technology, an outcome that relaxing legal barriers is unlikely to achieve.
The rapid spread of the COVID pandemic, the vast quantities of products needed, and the complexity of producing highly engineered products should raise the question of whether relaxing patents might achieve needed goals.
The prevailing approach to access is rooted in a world where international firm-to-firm technology transfer is accomplished via imitation or reverse engineering. But if imitation at scale is difficult, because of either characteristics of the manufacturing processes or the nature of the supply chains, then removing the legal barriers to market entry will be insufficient. The new market participants still need to acquire and absorb the relevant technology and knowledge—and for this they will need the originators’ assistance. Specifically, they will need technology transfer based on partnership, where originators teach the technology and share tacit knowledge and know-how, and in doing so help their partners learn to do things better and more quickly than would be possible if left to their own devices.
A further challenge that COVID presents for the prevailing approach is the importance of time. Massive scaling up of production must occur quickly. Every month spent waiting for an effective vaccine, for example, is another month where global life is fundamentally disrupted.
To understand how such partnerships could speed up manufacturing of complicated pharmaceuticals, it’s helpful to consider another medical technology. Early in the pandemic, General Motors dedicated resources to producing ventilators, specifically a complex model that was designed and produced by Ventec, a manufacturer based in Washington state that was also looking to ramp up production. As recounted in the New Yorker, a team of GM engineers flew to Washington to learn the manufacturing process from Ventec’s staff, returning to their home factory armed with pictures and videos. A few dozen Ventec engineers also travelled in the other direction, to GM, essentially on loan to help the auto company absorb the technology and set up production.
When non-originator “generic” pharmaceutical firms set out to produce drugs, by contrast, their scientists and engineers don’t travel to originators’ facilities to absorb technology and knowledge. They don’t need to: the companies are skilled at reverse engineering drugs and scaling up production on their own. Technology transfer occurs via imitation, not partnership.
To be sure, GM, with its immense engineering talent, probably could have acted like a generic drug firm and reverse-engineered Ventec’s ventilator on its own—but it would have taken much longer and been more prone to error.
Whereas small-molecule drugs developed via traditional processes of chemical synthesis are generally easy to produce, doing so for biologic drugs and vaccines is more difficult. These products, based on living, biological, and genetic resources, consist of larger and more complex molecules that are derived through more elaborate manufacturing processes. Many vaccines in particular rely on highly sophisticated technological platforms. Furthermore, vaccines, which are given to whole populations, require volumes of output far higher than the biologic drugs used to treat only the ill.
During a pandemic, time matters, which is why the partnership model used to make ventilators makes a great deal of sense as a way to speed up production of complex pharmaceuticals, particularly when massive levels of output are needed, as is the case with vaccines. Conceptually, the matrix below provides a way of thinking about the process in terms of greater or lesser degrees of two dimensions: the difficulty of replication at the required scale, and the importance of speed.
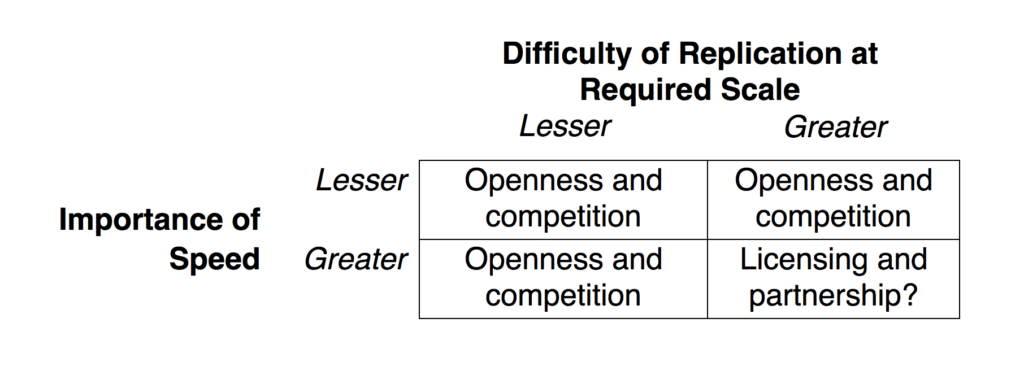
For most pharmaceutical products, in most times, maximizing access can best be achieved via openness and competition. This includes treatments that are easier to produce, such as generics, or more difficult, such as biosimilars. And when there is less urgency, this category also includes vaccines—where production and scale can be achieved with time. It also includes, even in a pandemic, products that can be replicated at the required scale (millions rather than billions). But in exceptional circumstances, it may be that the opposite of openness and competition—licensing and partnership—are best suited to achieving wide access quickly.
In these exceptional circumstances, the usual approach of relaxing legal barriers to create openness and competition may prove insufficient, because potential competitors will struggle to replicate at scale quickly enough when left to their own devices. Such openness could actually be counterproductive, were it to discourage companies from collaborating with partners in the necessary ways, actively sharing knowledge, technology, and expertise.
Whereas small-molecule drugs developed via traditional processes of chemical synthesis are generally easy to replicate, doing so for biologic drugs and vaccines is more difficult.
Nor is it easy to see how this sort of collaboration can be mandated. It’s one thing to allow Firm A to use Firm B’s proprietary knowledge and technology without B’s permission, when A can readily acquire these on its own; that’s what relaxing patents makes possible. It’s an entirely different matter to require, mandate, or compel B to actively share and teach its knowledge and technology.
As can be seen from this argument, although much of the discussion around pharmaceutical patents typically focuses on innovation incentives, the real issue now is about technology transfer and production. It may be that patents, rather than getting in the way, can provide the basis for partnerships, and thus the backbone of the network of licensees and manufacturing necessary for scaling up production.
In sum, for products that are difficult to replicate at the required scale and where it is urgent to produce a lot in short periods, openness and competition among multiple suppliers may be less desirable than having patent holders organize networks of licensees and manufacturers, letting them coordinate and manage global supply chains, like giant, vertically integrated firms.
While making this case for the potential benefits of patents for achieving access in the midst of the pandemic, it is essential to underscore that this is an exceptional situation. The research-based pharmaceutical industry regularly makes similar claims about its products, and also argues that its innovation is maximized by allowing originators to retain control and pick their licensees and partners. It’s a convenient argument for firms whose business model depends heavily on retaining intellectual property rights; it suits their interests to depict the relaxation of patents as being counterproductive in all instances. But the proposal offered here does not endorse that. Outside the exceptional circumstances marked by the bottom-right cell of the matrix, there are sound reasons to regard openness and competition as keys for expanding access. But the bottom-right cell needs to be taken seriously—even a broken clock will be right on occasion.
Following this approach will mean the world can’t simply sit back and watch pharmaceutical firms build their licensee and manufacturing networks, or count on them to altruistically supply products widely and cheaply. Firms should be encouraged—and given specific incentives—to license and share their technologies widely, and to build large networks of licensees to manufacture and distribute at scale. Funders, including governments, international agencies, and philanthropic organizations, can attach access conditions to their resources, demanding, for example, that companies charge affordable prices for their products and that production be sufficient for global distribution. And recipients of funding should be expected to make their licensing arrangements public, so policy-makers and the public can scrutinize the technology transfer components to determine whether they meet expectations.
Governments should also sharpen their policy and regulatory tools, to make sure that their patent systems provide officials with sufficient strength to negotiate prices, for example, and that their competition authorities are able to combat practices that restrict supply. Of course, some observers may question the utility of using such tools, which are designed to promote competition and openness, on the grounds that they would be ineffective or even counterproductive. That is, there is the possibility that in demanding that firms do the right thing, governments’ only recourse will be to take measures that slow the process of scaling up production. But, again, the case for patent-based licensing and partnership is an exceptional one: not all drugs are so complex to replicate and require such scale of production that openness and competition are not the best way to achieve access, even during a pandemic. Thus, an access policy still requires governments to have useful policy and regulatory tool kits to use when appropriate.
Society can also think creatively about how having a tailored tool kit of access instruments can be useful for drugs in the bottom-right cell. Governments can signal to companies that if they don’t license, produce, and price responsibly now, then for future products, when production at scale is less difficult or when time is less of a concern, regulators will be prepared to act decisively and aggressively to secure lower prices. Companies need to know that if they don’t do the right thing now, they risk punishment later.
The primary focus should be on promoting the transfer of knowledge and technology, an outcome that relaxing legal barriers is unlikely to achieve.
The logic of this temporal approach to incentives is similar to one used in the US Food and Drug Administration’s priority review voucher program, where a firm that introduces a new drug to address designated, unmet needs is rewarded with additional commercial life on a subsequent product. Thus, this program signals to firms that if they do the right thing early on by innovating for neglected disease, they can make higher-than-expected profits on a later drug through expedited review. In the case of COVID drugs, the message would be that firms that do not do the right thing now by licensing, producing, and pricing their products in ways that generate sufficient and affordable supply can expect lower-than-expected profits on later drugs.
Finally, developing countries may wish to invest substantially in national or regional production capabilities, enabling local firms to participate in technology partnerships as licensees. Such investments can potentially allow more local firms in at least a set of key countries, and possibly across the Global South, to learn to reverse-engineer and replicate complicated drugs and other products—an outcome that would greatly facilitate meeting the challenges of providing needed amounts of therapies and vaccines worldwide. After all, if there were hundreds of labs ready to create reverse-engineered versions of complex products (or could quickly become ready to do so), then the most important barriers to increasing supply would be primarily legal rather than technical, as was the case with HIV/AIDS 20 years ago.
These concerns are not entirely new. The World Trade Organization’s Agreement on Trade-Related Aspects of Intellectual Property Rights and the World Health Organization’s 2011 Global Strategy and Plan of Action on Public Health, Innovation and Intellectual Property address technology transfer. And of course the WHO’s C-TAP is, at its core, a global initiative for technology transfer. But insufficient attention has been given to how best to achieve this. Calling for technology to be transferred to multiple producers asks as many questions as it answers. It is necessary to move beyond the small-molecule narrative and approach, where technology transfer via imitation is the expectation. For some products, effective technology transfer requires the active participation of the proprietor, something that is difficult to force or compel.
My goal here is to summon the community of analysts concerned with access to medicines to take much more seriously the challenges involved in manufacturing complex and hard-to-replicate pharmaceutical products at large scale, and to acknowledge the important role that firm-to-firm international technology transfer that is accomplished via licensing and partnership can play. Governments and the private sector need to develop creative approaches to encourage, consolidate, and institutionalize this sort of technology transfer.
